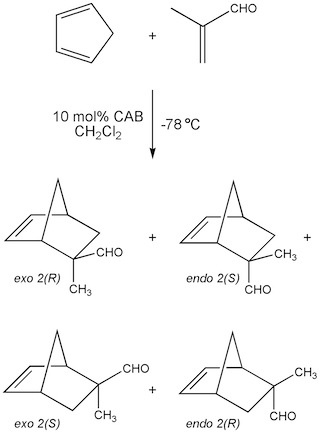
Asymmetric Diels-Alder reaction of cyclopentadiene and methacrolein. The chiral acyloxyborane catalyst (CAB) helps control the stereochemical outcome of this cycloaddition reaction. (Image by MIT OpenCourseWare.)
Instructor(s)
Prof. Rick Danheiser
Prof. Timothy Swager
MIT Course Number
5.37
As Taught In
Spring 2009
Level
Undergraduate
Course Description
Course Features
Course Description
This course, which spans a third of a semester, provides students with experience using techniques employed in synthetic organic chemistry. It also introduces them to the exciting research area of catalytic chiral catalysis.
This class is part of the new laboratory curriculum in the MIT Department of Chemistry. Undergraduate Research-Inspired Experimental Chemistry Alternatives (URIECA) introduces students to cutting edge research topics in a modular format.


